In response to the COVID-19 outbreak, Imperial’s experts in medical device design (bioengineering) – and a clinician treating infected patients – have designed an emergency ventilator that can be built to meet MHRA and FDA requirements using generic parts. JAMVENT provides a simple, low-cost solution to ventilator shortages worldwide, particularly for health services in developing countries. Plus the robust design also makes it suitable for long term use beyond current COVID-19 needs.
The design document for the JAMVENT ventilator can be requested by those wishing to develop ventilators for their local healthcare providers and includes a list of parts and a software spec.
We welcome enquiries from health organisations, manufacturers and donors interested in working with us to take JAMVENT from design to manufacture.
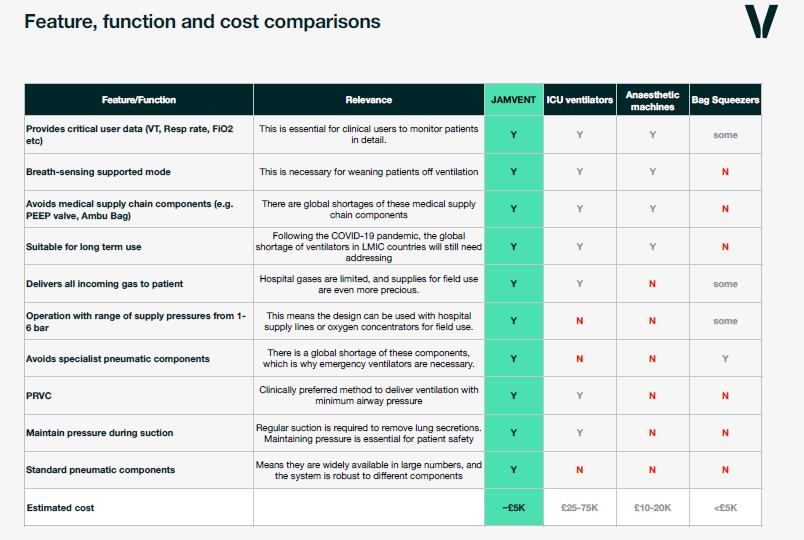
Design benefits
- Long term use – not specific to the COVID-19 crisis
- Easy to manufacture from low cost components (estimated at around £1,500 in the UK)
- Does not require specific pressure transducers or solenoids
- Parts can be sourced from various manufacturers – avoiding supply-chain bottlenecks
- No specialist or medical supply chain components – and no balloons
- Simple electronics
- Doesn’t require gas to drive it – unlike some others – which is critical where gas supply is limited.
- Employs fluid mechanical design principles
Ventilator design meets clinicians needs
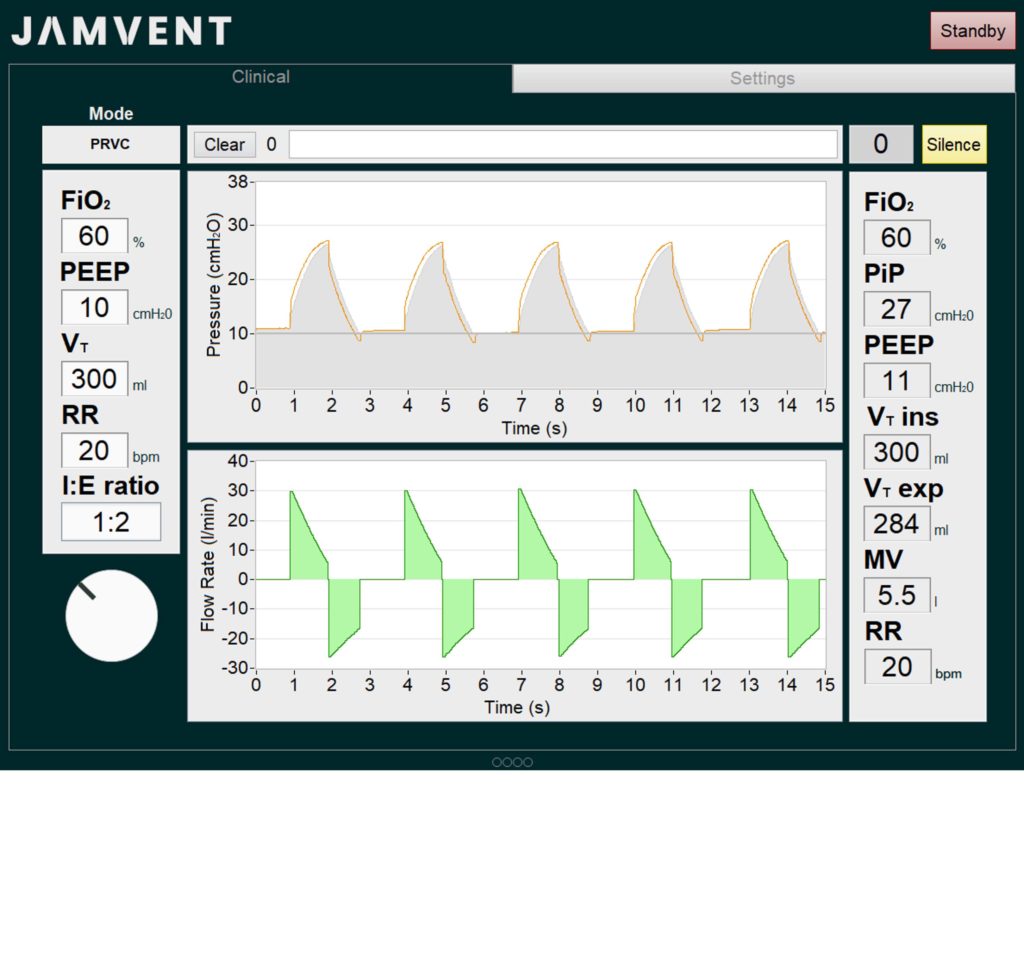
- PEEP controllable electronically
- Pressure Regulated Volume Control (PRVC) mode
- Supports spontaneous breath
- Maintains PEEP during suctioning
Performance evidence
Evidence that the JAMVENT prototype can perform to MHRA and ISO 80601 tests can be downloaded here.
- JAMVENT Evidence of Performance V2- MkIV prototype and ISO 80601 tests
- JAMVENT Evidence of Performance V1 -MkIII prototype and MHRA RMVS tests
It also highlights PRVC and spontaneous mode performance and how it can maintain PEEP during suctioning – critical functions of ICU ventilators for COVID-19 patients.
JAMVENT ventilator in action – click to view a video
- Dr Sherwood presents the Mark II prototype and describes the basic components and their functions
- Dr Sherwood takes a look inside the Mark III prototype and describes the basic components and their functions
- Dr Joseph Sherwood and Dr Jakob Mathiszig-Lee demonstrate JAMVENT in PRVC mode, and the rapid response to a change in User Inputs. The mock lung has a resistance of 20 cmH2O/(l/s) and compliance of 30 ml/cmH2O.
- Dr Mathiszig-Lee and Dr Sherwood demonstrate JAMVENT in spontaneous mode, where the system senses breath from the patient and provides a supported breath. When no breath occurs for 20 seconds, PRVC mode restarts automatically.
- Dr Mathiszig-Lee demonstrates JAMVENT in suction mode. A suction of 30 l/min is applied next to the entry to the mock lung. The system maintains pressure in the range 5-6 cm H2O for significantly longer than the 3 seconds required by the test in the MHRA guidelines.
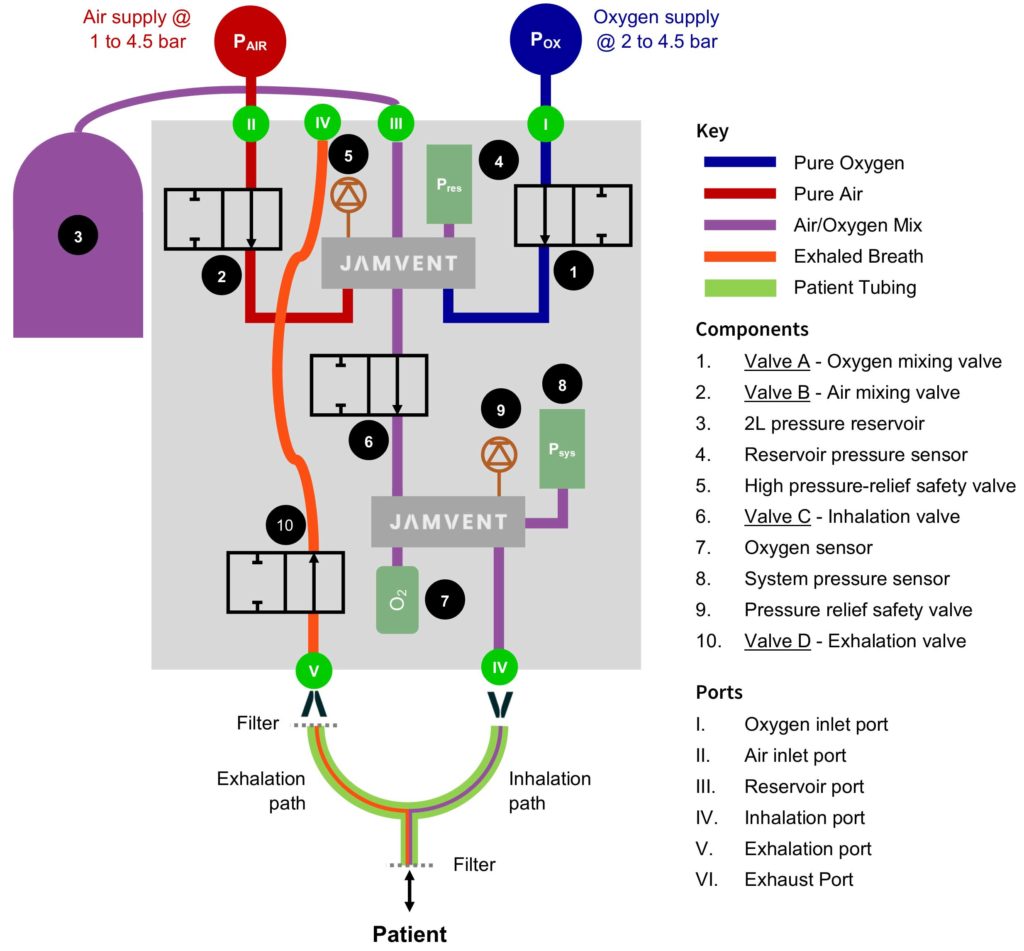
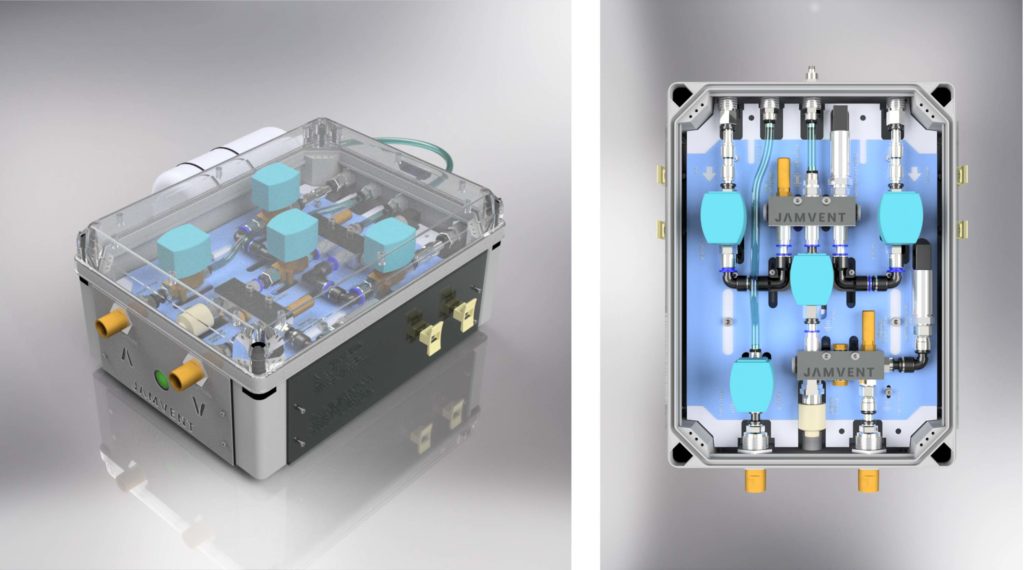
Ventilator system

Creators of the emergency ventilator design
- Project and Technical Lead – Dr Joseph Sherwood – RAEng Research Fellow, Department of Bioengineering. Research areas include biofluid mechanics, flow measurement/control, device design for research.
- Clinical Lead – Dr. Jakob Mathiszig-Lee – Honorary Research Fellow and Senior Anaesthetic Registrar at the Royal Brompton Hospital. Department of Surgery and Cancer.
- Project Manager – Prof James Moore – The Bagrit Chair in Medical Device Design, Department of Bioengineering. Research areas include biofluid mechanics, cardiovascular device design, device translation.
- Co-Technical Lead – Dr Michael Madekurozwa, Department of Bioengineering. Research areas include experimental biofluid dynamics, design and implementation of tools, hardware and software for use in research.
Who to contact:

Vibhor Jajoo
Engagement Manager
For Chemical Engineering, Earth Sciences, Bio-Engineering, Bio-Tech and Medical Devices.